How do we decide which fluorophores to use for spectral flow cytometry? Last week I suggested treating spectral cytometers just like conventional cytometers with lots of detectors. For the purpose of fluorophore selection, this means picking one fluorophore to peak in detector. I'll show some exceptions to this rule, and also show how they aren't as exceptional as they might first appear. The guidelines I'm presenting here are most important as you increase the size and complexity of your panels; as you approach the limits of the instrument, focusing on these principles will give you greater benefits.
Today let's look at how to go about determining which fluorophore might be best for each detector. What we want to achieve is the best signal to noise ratio so that we can cleanly see our markers with minimal interference. Broadly, this means selecting fluorophores with narrow peaks and limited off-peak signals. Let's look at some examples to show what I mean.
Here's PerCP-Cy5.5 as it appears on the 5L Cytek Aurora:

Plot of PerCP-Cy5.5
Typically, the peak emission occurs in the B9 (Blue 9) channel. However, we can also see quite strong emission (over 60% in some cases) off the violet laser (V12 & V13), yellow-green (YG5, YG6, YG7) and red (R3, R4), with less on the UV (UV12-14). PerCP is very good at collecting photons.
These off-peak emissions are going to cause spread in those areas of the spectrum. What's in those areas?
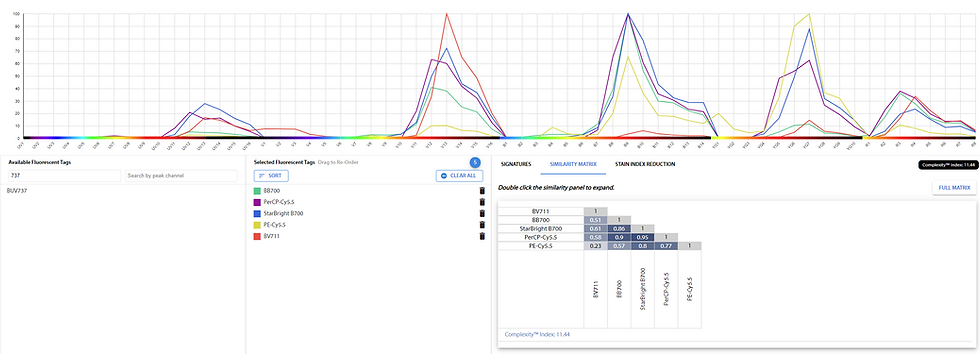
Plot of PerCP-Cy5.5, BV711, PE-Cy5.5, BUV737
Well, it's BV711/SB702/SBV710 in the violet, PE-Cy5.5 or PE/Fire-700 (also PE-Cy5) in the yellow-green, and Spark NIR 685 or AF660 (also AF700 or R718) in the red. So, we would want to consider designing our panel such that PerCP-Cy5.5 is on a different set of cells than PE-Cy5.5, BV711, possibly BUV737 and Spark NIR685. That makes our panel design harder. Alternatively, we limit our spread by keeping the PerCP-Cy5.5 signal in check, as described in this post.
Is there a better solution? Absolutely. PerCP-Cy5.5 is an older fluorophore and a tandem to boot. If we search for other fluorophores peaking in B9, we see BB700 and Star Bright Blue 700. SBB700 has less red emission, while BB700 has less yellow-green and violet with a narrower peak in the blue. Additionally, both BB700 and SBB700 are considerably brighter than PerCP-Cy5.5.

Plot of PerCP-Cy5.5, BB700 and SBB700
Similarly, here's a comparison between PerCP-eFluor710 and RB705:

Plot of PCPe710 (pink) and RB705 (green)
RB705 essentially eliminates spillover into the violet and yellow-green with a reduction in red as well. There is a tradeoff in that the peak in the blue is wider, so we would expect more interference between our B9 dye (say BB700) and our B10 dye (in this case RB705). From a panel design perspective, it's easier to deal with one clash between immediate neighbors rather than clashes across all lasers.

Plot of BB700 (blue) and RB705 (green)
The main limitations of moving to newer fluorophores are cost and fewer options for conjugates. Optimizing for cost is fairly simple: for the most expensive fluorophores, pick targets where you know you can use the antibody at a higher dilution factor. This will reduce your cost per stain. Pick the newest fluorophores first for your panel since these have restricted options; fill in the slots with many options later.
Exceptions (sort of) to the one fluorophore per detector rule:
There are fluorophore combinations that can be unmixed adequately even in 50 color panels while violating this principle of one fluorophore per detector. Let's look at some examples and see why these work.
First, we have combinations in V8. The published combinations are using BV570 and Pacific Orange:

BV570 vs. Pacific Orange
These are both terrible dyes in my opinion. They are messy and very, very dim.
Here's where we'll see interference from these dyes:

What can we use instead? SBV570 is a bright dye with less spillover into PE. Spark Violet 538 is brighter than Pacific Orange, but just as messy. Qdot 545 is an alternative that has very narrow emission, but is only available as streptavidin or a conjugation kit. Because of this, you also won't find it in most spectrum viewer tools.

Star Bright Violet 570

Spark Violet 538

Qdot 545
Okay, so we can use these together even though they share a peak emission channel--why? If you look at the spectra, you can see that while BV570/SBV570 has a relatively narrow peak in V8, Pacific Orange and Spark Violet 538 have over 90% signal appearing in V7. So, these are basically V7 or V8 dyes, and we can think about it as them being "assigned" to V7 for the purposes of unmixing. Similarly, Qdot 545 has very strong UV signal in a channel where no other dyes will emit--this means we can very clearly distinguish Qdot 545 from everything else by using the UV8 channel.
This is effectively the same as what we do on a conventional cytometer if we use PE-Cy5.5 and PerCP-Cy5.5 in the same panel: the signal of PE-Cy5.5 in the PerCP-Cy5.5 channel will actually dominate because PE gets excited so well by the blue laser. We have two channels to work with, though, so we can assign the signals to a channel per fluorophore and reduce interference via compensation.
What we can't do is use pairings like this for co-expression.
APC/Fire-810 and Alexa Fluor 790 is another pairing that works this way:

APC/Fire 810 (blue) + AF790 (red)
Why does this one work? AF790 has a near-100% peak in V16. You won't find any other fluorophore peaking in V16. AF790 is really, really dim, though because it prefers to be excited by an infrared laser. This combination doesn't work very well on the Discover S8 where that violet peak is quite a bit lower.
The final type of pairing that can be unmixed despite peaking in the same detector is the "shoulder". These are tougher to get to work well. Here are two examples:

Star Bright UV 795 (lilac) and BUV805 (pale blue)

Real Blue 780 (blue) and PerCP/Fire-806 (green)
We can unmix these provided there's no other fluorophore peaking in the "shoulder" channel (that's UV15 for SBUV795 and B13 for RB780). These work, but the distortion and spread between the two can be difficult to work with. These will work better if your UV and blue detectors have empty channels or if you don't have a fluorophore peaking in V16, where both SBUV795 and PerCP/Fire-806 have secondary emission. That will allow the unmixing to gain additional information to resolve the pairings.
Note that in these "shoulder" situations, the fluorophore that suffers the greater loss of resolution is the one that gets "leaned on", i.e., the one with the longer emission wavelength. This is also true for the symmetrical situation with the shoulder up against the narrow end of the detector limit (think BB515 and Kiravia Blue 520 or BV421 and Star Bright Violet 440); again, while there is unmixing distortion and spread in both directions, the resolution of the fluorophore with the longer average emission wavelength suffers more. This is due to the hard upper bound of emission due to quantum mechanics and conservation of energy. Shorter wavelengths have more energy. There's a limit to how much energy can be conserved in the emission, leading to an asymmetrical emission distribution with a longer tail towards the longer, lower energy wavelengths (cases like RB780 where we don't see this shape are because we don't measure the longer wavelengths). So, error and spread are more likely to propagate down rather than up the spectrum.

Comments